Thermochemistry of the system K2O-CaO-SiO2 (2020-2024, FWF project P30754)
In their Energy 2020 agenda the EU defined several ambitious goals such as increasing the amount of energy produced from renewable sources by 20% and simultaneously reducing greenhouse gas emissions by 20%. Furthermore, the EU is committed that moving to a competitive low-carbon economy is completed by 2050. Solid biomass is considered to be an important sustainable energy source for meeting these targets. However, the combustion or gasification of biomass such as firewood, agricultural waste, crop stalks or straw for the production of heat and electricity results in enormous amounts of ashes and/or slags for which utilization options are eagerly required and studied. Just to give a number for Austria, a recent report on the biomass ash flows issued in 2016 by the federal environment agency for the base year 2013 evaluated a total volume of about 133.000 t biomass ash. On the other hand, the formation of slags and ashes has also implications for process control since they can cause severe problems in practical operation and may in the worst case result in an unscheduled shutdown of the whole biomass-fired power/heat generation plant.
Within this context the system K2O-CaO-SiO2 comes into play because it is the main subsystem of silicate/oxides ashes in biomass and can be used as a model system for the interpretation of the relevant high-temperature processes and reactions. The last comprehensive and frequently used study of the phase equilibria in this system has been performed back in the 1930’s. Recent investigations of our group show that considerable suspicion has to be attached not only what concerns the number of phases but also what concerns their chemical compositions and melting characteristics. Therefore, it is not that surprising that the prediction of ash and slag behavior during biomass combustion that was based on the previous study turned out to be imprecise and error-prone.
The central goal of this project is a complete re-investigation of the ternary system K2O-CaO-SiO2 . An important innovative aspect will be the combination of experimental equilibrium studies with measured thermodynamic properties and state-of-the art thermodynamic modeling within the same project. For this purpose the following key questions will be addressed: (1) How many solid compounds (phases) do exist and how is their melting behavior? (2) What are their melting points and heat capacities? (3) What are the solid state properties of the compounds including their crystal structures? (4) Is it possible to model thermodynamic properties of the system based on the novel experimental data? (5) How good is the agreement between the predicted and measured thermodynamic characteristics? Generally speaking, the proposal tries to bridge the gap between
fundamental research on a ternary oxide system and applied science related to biomass combustion and technical mineralogy.

Abb. 1: Pulverbeugungsdiagramm der Phase K4Ca6Si6O15

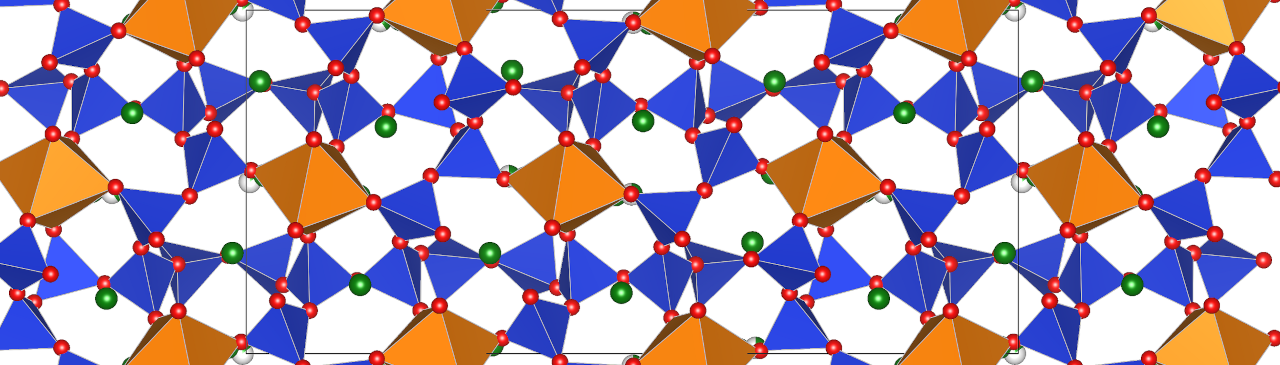
Abb. 2: Kristallstruktur der Phase K4Ca6Si6O15 als Projektion längs [100]: SiO4-Tetraeder: blau; CaO6-Oktaeder: orange; K-Kationen: grün
Publications
Hang, L., Hildebrandt, E., Krammer, H., Kahlenberg, V., Krüger, H. & Schottenberger H. (2021): K4CaSi6O15 – Solving a 90-year-old riddle. J. Am. Ceram. Soc. 104, https://doi.org/10.1111/jace.17920.