Seminar of the Department of Microbiology
MSc Student Session 3
Sabine Oberhofer - University of Innsbruck, AG Neuhauser
Katharina Russ - University of Innsbruck, AG Kirchmair
Leonie Sonderegger - University of Innsbruck, AG Podmirseg
01.12.2022, 11:00 - Hybrid - Join online - or in presence: Seminarraum Mikrobiologie (Bauteil V)
Abstracts
Oberhofer: Phenotypical assessment of Plasmodiophora brassicae infection of different Arabidopsis arenosa ecotypes
Plasmodiophora brassicae is a soil-borne obligate parasite (Kageyama and Asano, 2009), which causes clubroot disease in brassicas (Faggian and Strelkov, 2009). Infections with P. brassicae can lead to gall formation in the roots and hypocotyl, causing reduced growth of the host plant (Faggian and Strelkov, 2009) and annual crop losses of 10 % worldwide (Dixon, 2006). It is assumed that all members of the Brassicaceae family can be potential hosts for P. brassicae (Dixon, 2009). Arabidopsis arenosa (Brassicaceae) is a representative of the model genus Arabidopsis with diploid and autotetraploid populations, living in alpine and lowland areas and showing pronounced phenotypical differences among them (Monnahan et al., 2019). The aim of this master thesis is the phenotypical characterization of the parasite-host interactions of P. brassicae with different ecotypes of A. arenosa. Phenotypical data were generated by infecting different A. arenosa ecotypes with P. brassicae. Additionally, molecular and physiological methods, such us PCR and qPCR, and hormone measurements via GC-MS; were performed to quantify and investigate the infection process. This presentation gives first insights on the phenotypical characterisation of P. brassicae infection on wild populations of A. arenosa.
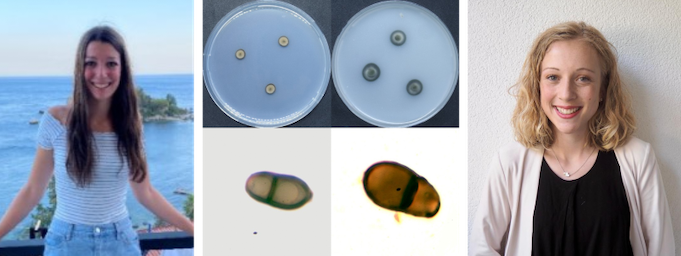
Russ: The hidden diversity of black yeasts involved in the carton nests of Lasius fuliginosus (Hymenoptera: Formicidae)
The present study examined the fungal core mycobiome of Lasius fuliginous carton nests. Three different nests were sampled in September and October 2021, the first two nests are located in Innsbruck, Tyrol in Picea abies and the third nest was from Konstanz, Baden-Württemberg in Acer platanoides. Trees were sampled using a drill to get to the nest carton material inside the tree. For the isolation of the fungi in the carton nest, the dilution to extinction method was used. This cultivation method allowed 355 pure cultures to be isolated, which were then further analysed with molecular biological methods and phylogenetic analyses using a Maximum Likelihood (ML) tree.
All the isolated fungi belonged to phylum Ascomycota. The most found order was Chaetothyriales (Eurotiomycetes), also known as black yeasts, containing the genera Cladophialophora, Exophiala, Phialophora and Cyphellophora. Also, fungi from the genus Ochroconis (Venturiales) were found. These two orders accounted for the most commonly isolated fungi, resulting in the core mycobiome of the fungal partners of the Lasius fuliginosus carton nests (Chaetothyriales 67.6 %, Venturiales 23.3 % of the total fungi). Fungi of the genera Lophiostoma and Nigrograna (Pleosporales), and Rhizodiscina (Aulographales) were present in low abundance. Phylogenetically, the majority of the Chaetothyriales isolates of the sampled nests clustered with or close to fungi of other carton nests. The Venturiales clustered in two different clades with fungal isolates of other Lasius fuliginosus carton nests. For the first time there is a clear insight in the fungal core mycobiome of Lasius fuliginosus carton nests. Carton nests of this ants are a high diversity habitat, resulting in as of yet undescribed fungal species. Further studies may look deeper in this mutualism and also include the bacterial community.
Sonderegger: Live and let die – Resting structures of anaerobic fungi
Anaerobic Fungi (AF) of the phylum Neocallimastigomycota are important inhabitants and plant matter degraders of the digestive tract of ruminants and hindgut fermenters. The lifecycle of AF is indeed particular as they have a motile and a vegetative stage. In principle, the motile zoospores attach themselves to plant material, germinate, form rhizoidal structures, and build a sporangium which is again filled with zoospores. However, some studies suggest an additional stage, a resting structure (RS) that might function as a yet unknown aero-tolerant life phase. This would explain why AF can be isolated from fresh, dried, or frozen feces even after several months of storage. Furthermore, in different AF taxa, resting cysts, melanized resistant sporangia, and multi-chambered spore-like structures have been described. In this study, we wanted to monitor their responses to environmental conditions and induce RS formation in AF to elucidate this missing link of the AF life cycle. For that, three morphologically different AF strains were selected and subjected to three RS trigger scenarios.
