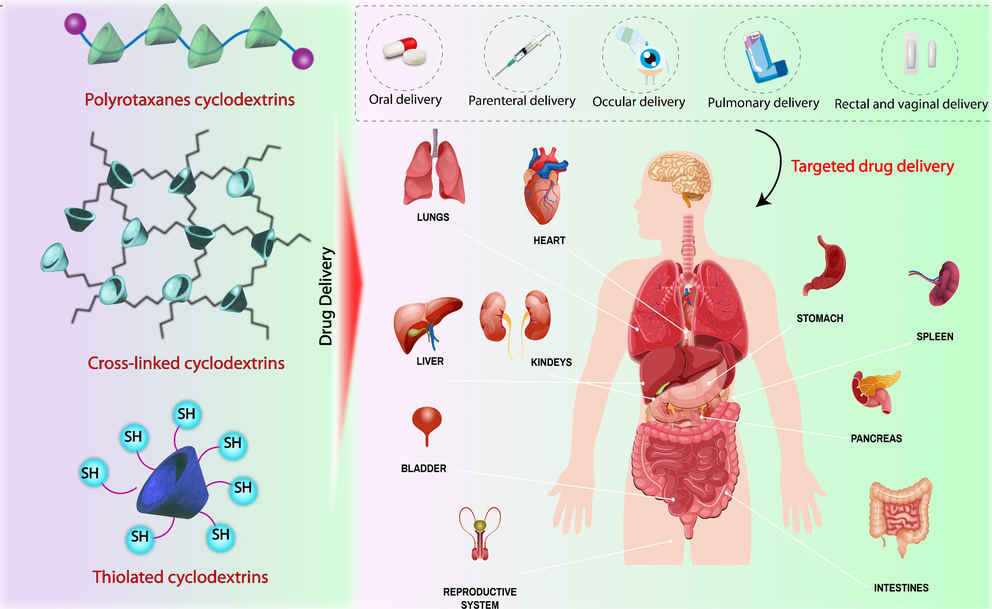
Research Topics
In our lab, we focus on developing novel oligomeric and polymeric drug delivery systems.
We synthesize various thiolated cyclodextrins and polysaccharides primarily through two reaction pathways:
A high degree of thiolation can be achieved through reaction with phosphorus pentasulfide. With this method, up to complete thiolation can be reached.
S-acetyl-mercaptosuccinic anhydride forms ester bonds with hydroxyl groups, offering both thiolation and high aqueous solubility.
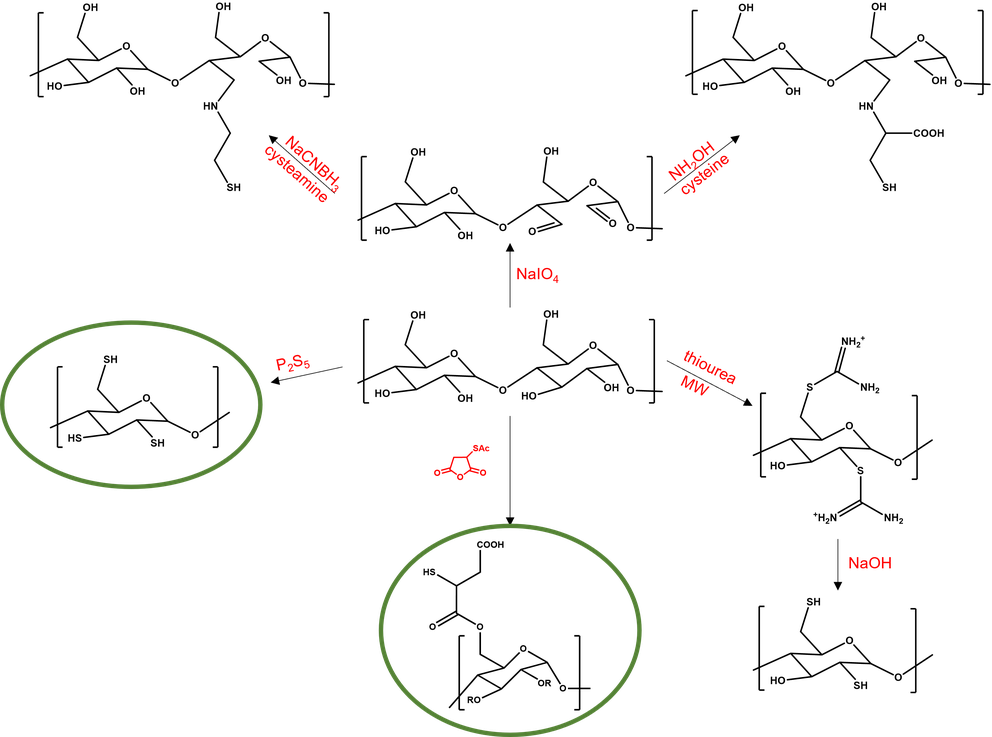
Other modified oligosaccharides and polysaccharides, such as cationic or unsaturated ones, are also the focus of our interest.
Relevant publications:
Kali, G.; Knoll, P.; Bernkop-Schnürch, A. Emerging technologies to increase gastrointestinal transit times of drug delivery systems. J. Control. Release 2022, 346, 289-299
Grassiri, B.; Cesari, A.; Balzano, F.; Migone, C.; Kali, G.; Bernkop-Schnürch, A.; Uccello-Barretta, G.; Zambito, Y.; Piras, A.M. Thiolated 2-Methyl-β-Cyclodextrin as a Mucoadhesive Excipient for Poorly Soluble Drugs: Synthesis and Characterization. Polymers 2022, 14, 3170.
Kali, G.; Haddadzadegan, S.; Laffleur, F.; Bernkop-Schnürch, A. Per-thiolated cyclodextrins: Nanosized drug carriers providing a prolonged gastrointestinal residence time. Carbohydr. Polym. 2023, 300, 120275
Fürst, A.; Kali, G.; Efiana, N.A.; Akkuş-Dağdeviren, Z.B.; Haddadzadegan, S.; Bernkop-Schnürch, A.; Thiolated cyclodextrins: A comparative study of their mucoadhesive properties. Int. J. Pharm. 2023, 635, 122719
Kaplan, Ö.; Truszkowska, M.; Kali, G.; Knoll, P.; Blanco Massani, M. Braun, D. E.; Bernkop-Schnürch, A.; Thiolated α-cyclodextrin: The likely smallest drug carrier providing enhanced cellular uptake and endosomal escape. Carbohydr. Polym. 2023, 316, 121070.
Haddadzadegan, S.; Knoll, P.; Wibel, R.; Kali, G.; Bernkop-Schnürch A.; Three generations of thiolated cyclodextrins: A direct comparison of their mucus permeating and mucoadhesive properties. Acta Biomater. 2023, 167, 309-320.
Kali, G.; Fürst, A.; Efiana, N.A.; Dizdarević; A.; Bernkop-Schnürch, A.; Intraoral Drug Delivery: Highly Thiolated κ-Carrageenan as Mucoadhesive Excipient. Pharmaceutics 2023, 15(7), 1993.
Kali, G.; Özkahraman, B.; Laffleur, F.; Knoll, P.; Wibel, R.; Zöller, K.;Bernkop-Schnürch, A.; Thiolated Cellulose: A Dual-Acting Mucoadhesive and Permeation-Enhancing Polymer. Biomacromolecules 2023, 24(11), 4880–4889.
Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2023, 121500.
Kali, G.; Mahmoud Taha, A. M. M.; Campanella, E.; Truszkowska, M.; Haddadzadegan, S.; Denora, N.; Bernkop-Schnürch, A. Enhanced Mucoadhesion of Thiolated β-Cyclodextrin by S-Protection with 2-Mercaptoethanesulfonic Acid. ACS Omega 2024, 9(5), 5819–5828.
Fürst, A.; Kali, G.; Dizdarević, A.; Stengel, D.; Bernkop-Schnürch, A. Mucoadhesive polymers: Design of S-protected thiolated cyclodextrin-based hydrogels. Int. J. Pharm. 2024, 656, 124075.
Haddadzadegan, S.; Saleh, A.; Veider, F.; Knoll, P.; Laffleur, Kali, G.; Bernkop-Schnürch, A. Cyclodextrin-mediated enhancement of gastrointestinal drug delivery: unveiling mucoadhesive and mucopenetrating synergy J. Drug Deliv. Sci. Technol., 2025,
Davoudi Z.; Kali G.; Braun, D.E.; Azizi, M.H.; Bernkop-Schnürch, A. Highly thiolated corn starch for enhanced mucoadhesion and permeation Int. J. Pharm., 2025, 680, 125798
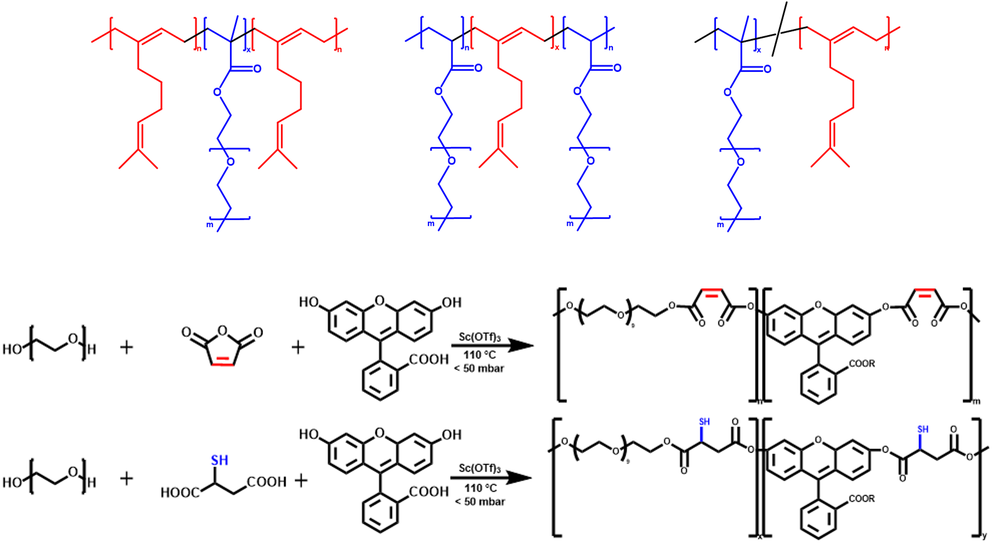
Our work focuses on novel synthetic polymer-based drug delivery systems.
- Polymerization of myrcene, a monoterpenoid, results in a controlled microstructure and beneficial properties as a drug delivery system. Additionally, these polymers also exhibit antioxidant activity. Therefore, we refer to them as Active Pharmaceutical Excipients.
- Polycondensation of PEG with mercaptosuccinic acid or maleic anhydride, combined with fluorescein, produces a mucoadhesive polymer with in-chain polymerized drug. This material exhibits sustained drug release at the application site.
Relevant Publications
Hilschmann, J.; Kali, G. Bio-based Polymyrcene with Highly Ordered Structure via Solvent Free Controlled Radical Polymerization. Eur. Polym. J. 2015, 73, 363–373.
Bauer, N.; Brunke, J.; Kali, G. Controlled Radical Polymerization of Myrcene in Bulk: Mapping the Effect of Conditions on the System. ACS Sustain. Chem. Eng., 2017, 5,10084-10092.
Niedner, L.; Kali, G. Green Engineered Polymers: Solvent Free, Room‐Temperature Polymerization of Monomer From a Renewable Resource, Without Utilizing Initiator. ChemistrySelect, 2019, 4, 3495-3499
Kali, G.; Gintsburg, D.; Molnar, L.; Knoll, A.; Bernkop-Schnürch, A. Mucoadhesive poly(ethylene glycol)-based biodegradable polyesters with in-chain model drug. J. Drug Deliv. Sci. Technol., 2024, 102, 106332
We investigate the synthesis possibilities of polyrotaxanes through threading approach and rotaxa-polymerization. Our main focus is to examine how functional groups influence the cellular uptake of polyrotaxanes and to understand potential degradation pathways in the cytosol that lead to the release of macrocycles.
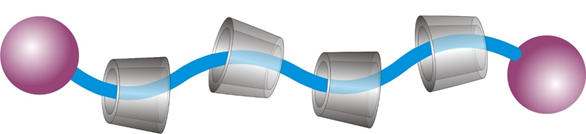
Relevant publications
Kali, G.; Eisenbarth, H.; Wenz, G. One-Pot Synthesis of a Polyisoprene Polyrotaxane and Conversion to a Slide-Ring Gel. Macromol. Rapid Commun. 2015, 37 (1), 67–72
Hilschmann, J.; Kali, G.; Wenz, G. Rotaxanation of Polyisoprene to Render it Soluble in Water. Macromolecules, 2017, 50(4), 1312–1318.
Hilschmann, J.; Wenz, G.; Kali, G. One-pot synthesis of block-copolyrotaxanes through controlled rotaxa-polymerization. Beilstein J. Org. Chem. 2017, 13, 1310–1315.
Wang, Y.C.; Maeda, R.; Kali, G.; Yokoyama, H.; Wenz, G.; Ito, K. Synthesis of Poly(Methyl Methacrylate)-Based Polyrotaxane via Reversible Addition–Fragmentation Chain Transfer Polymerization. ACS Macro Lett.2020, 9(12), 1853–1857
Kali, G.; Gintsburg, D.; Molnar, L.; Knoll, A.; Bernkop-Schnürch, A. Mucoadhesive poly(ethylene glycol)-based biodegradable polyesters with in-chain model drug. J. Drug Deliv. Sci. Technol., 2024, 102, 106332
Kali, G.; Mayer, A.H.; To, D.; Truszkowska, M.; Seybold, A.; Braun, D.E.; Plangger, R.; Gallei, M.; Bernkop-Schnürch, A. Polycaprolactone/α-cyclodextrin polyrotaxanes with cellular uptake enhancing properties. J. Mater. Chem. B, 2025,13, 3471-3482