One strategy for the treatment of solid tumours is the combination of radiotherapy and chemotherapy, known as radio-chemotherapy. Chemical agents can be used to prevent the tumour cells from growing by damaging their DNA, thereby enhancing the effect of radiotherapy. In the cells of hypoxic tumours there is a lack of oxygen. Since tirapazamine is only activated under oxygen-deficient conditions, the substance is particularly well suited for targeted damage to the corresponding tumour tissue.
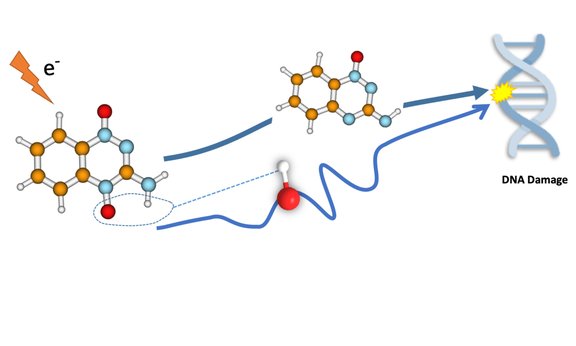
The mechanism of the drug follows an enzymatic reduction of the TPZ and the subsequent formation of different radicals. These radicals are important because they contribute to the damage of the tumour cell DNA and thus to the killing of the cancer. Until now, it was unclear how the respective radicals individually contribute to the biological efficacy of the substance. Two different radicals are combined to damage tumour cells. Besides the hydroxyl radical (OH radical with a single, unpaired electron), the benzotriazinyl radical, i.e. the oxidized radical of TZP, might be involved.
On the track of radical formation
The mode of action was investigated jointly by the group for computational photophysics of Milan Ončák and the group for inelastic electron scattering of Stephan Denifl at the University of Innsbruck with experimental work of PhD student Eugene Arthur-Baidoo. In their work, they dealt with the low-energy electron attachment to TZP and investigated the decomposition of the formed TPZ anion by mass spectrometry. Quantum chemical calculations then provided a detailed insight into the reaction dynamics. They observed that hydroxyl radical formation (HO.) is the main decomposition channel. In a special reaction pathway, the hydroxyl radical glides through the vicinity of the molecule and can attach itself to different parts of the molecule ("roaming mechanism"). In the most probable pathway, the hydroxyl radical leaves subsequently the anion.
The complementary reaction channel with the emission of a benzotriazinyl radical is almost insignificant. The situation changes when the tirapazamine molecules are microsolvated, whereby the production of hydroxyl radicals is considerably suppressed. The results obtained provide a clear picture of the basic molecular properties of this important molecule.