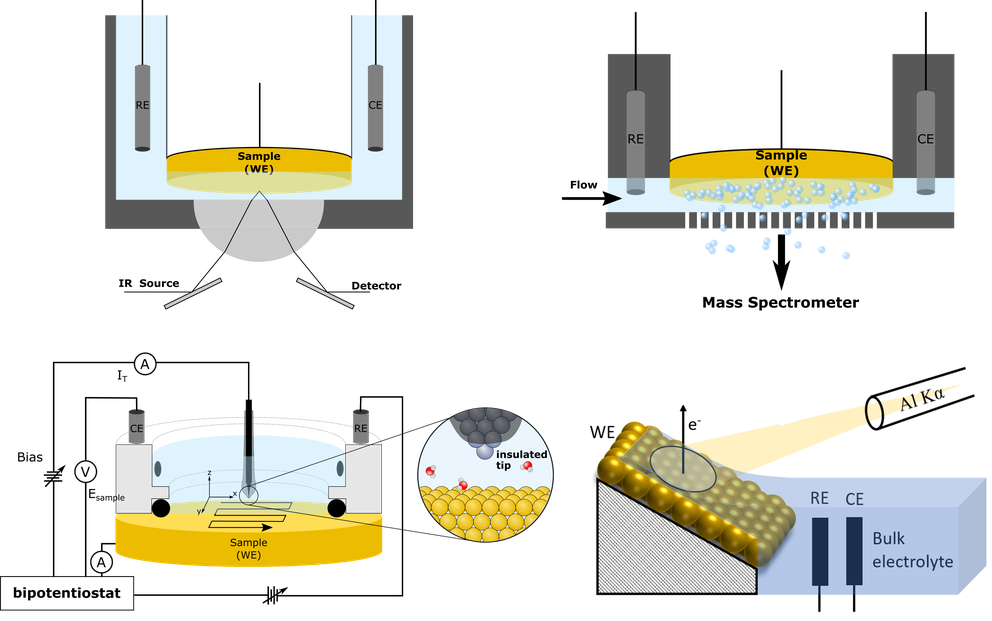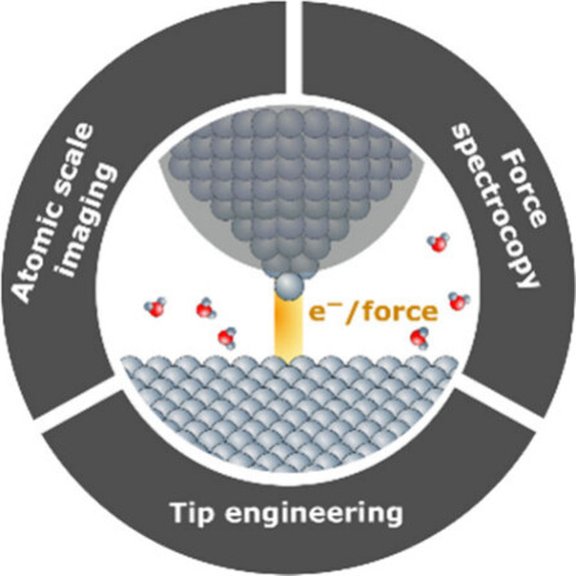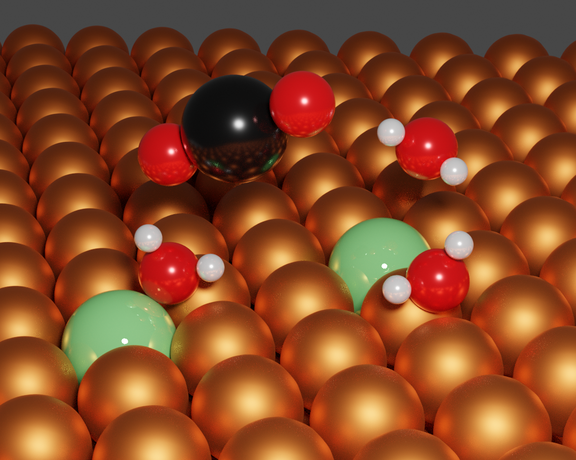
Approach:
- Material synthesis for the electrochemical CO2 reduction reaction (CO2RR)
- Gaining fundamental understanding of the CO2 and H2O activation at the solid/liquid interface
- In-situ analytics employed during electrochemical applications
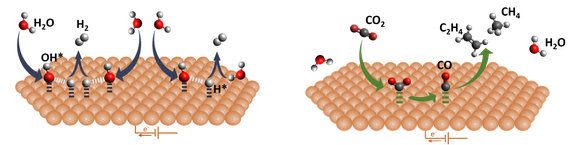
- Analysis of the elementary steps governing CO2 and H2O activation
- Direct comparison of the CO2 reduction (CO2RR) and hydrogen evolution (HER) pathways to uncover structure–reactivity relationships at the solid–liquid interface
- Mechanistic insights enabled by well-defined surfaces
01.08.2025
New Article Published in The Journal of Physical Chemistry C
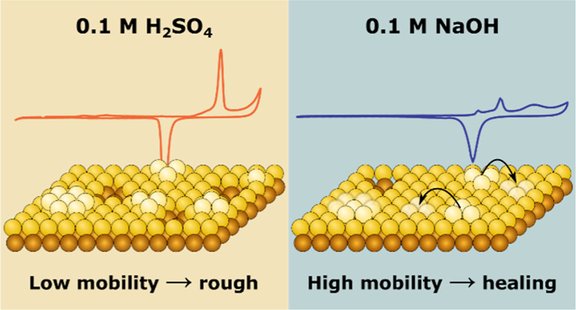
How do gold surfaces respond to repeated oxidation and reduction cycles? Our new article, "Dynamics of Adatom and Vacancy Islands on Au(111) in Alkaline and Acidic Media," investigates how the Au(111) surface evolves in acidic and alkaline solutions. The results reveal surprising differences in surface behavior and provide important insights into the self-healing properties of gold under different environmental conditions.
Link to the article: https://pubs.acs.org/doi/10.1021/acs.jpcc.5c03661
17.03.2025
New Article Published in Angewandte Chemie
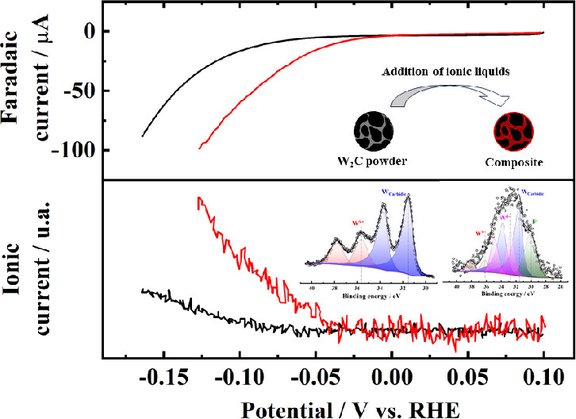
How does the surface of WC powder electrocatalysts change under real-world conditions? Our new article, "Surface Chemistry of WC Powder Electrocatalysts Probed In Situ with NAP-XPS," provides answers. Using the NAP-XPS technique, exciting insights into the dynamic processes occurring at the catalyst surface were gained — an important step toward the development of more efficient electrocatalysts.
Link to the article: https://onlinelibrary.wiley.com/doi/10.1002/anie.202500965
08.01.2025
New Article Published in Surface Science
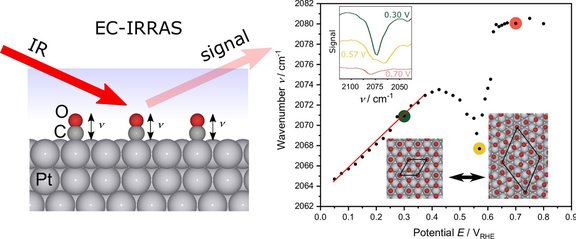
The so-called “anomalous” negative Stark effect of CO on Pt(111) has sparked debate in surface chemistry for years. In our new article, "Rationalizing the 'anomalous' electrochemical Stark shift of CO at Pt(111) through vibrational spectroscopy and density-functional theory calculations," we investigate this unusual behavior using advanced IR spectroscopy and DFT calculations. The study reveals surprising details about the structure and dynamics of CO adsorption at electrochemical interfaces.
Link to the article: https://doi.org/10.1016/j.susc.2025.122694
28.02.2025
New Article Published in ACS Nano
Our new article, titled "Combining Electrochemical Scanning Tunneling Microscopy with Force Microscopy" highlights recent advances in electrochemical scanning probe techniques. It introduces a combined EC-STM/qPlus-AFM approach that offers high precision and versatility for studying solid-liquid interfaces and outlines key future opportunities in interface characterization and electrocatalysis.
Link to the article: https://pubs.acs.org/doi/10.1021/acsnano.5c00591
22.04.2024
New Article Published in ACS Applied Materials & Interfaces
Our latest article, titled "Highly Active W₂C-Based Composites for the HER in Alkaline Solution: The Role of Surface Oxide Species," explores the role of surface oxide species in W₂C-based composite materials for the hydrogen evolution reaction (HER) in alkaline solution. The study demonstrates their high catalytic activity and provides valuable insights into the design of efficient HER catalysts.
Link to the article: https://pubs.acs.org/doi/10.1021/acsami.4c01612
08.01.2024
New Article Published in ACS Catalysis
The article "Electroreduction of CO₂ in a Non-aqueous Electrolyte – The Generic Role of Acetonitrile" provides an in-depth investigation of the role of acetonitrile as a solvent in the electroreduction of CO₂ in non-aqueous media, highlighting its influence on the efficiency of the reaction.
Link to the article: https://pubs.acs.org/doi/10.1021/acscatal.3c02700
Industrial Collaborations and Projects
Direct Carbon Capture and Electrolysis (DCCE)

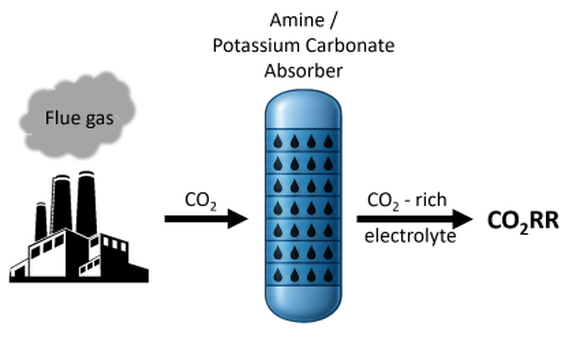
Approach:
- Direct CO2 capture from combustion plants followed by electrochemical CO2 reduction (CO2RR) to produce syngas (CO+H2)
- Application of in-house fabricated electrodes
- Electrochemical CO2RR tested with H-cell for fundamental understanding and full cell for practical application

- CO2 is captured with an amine solution
- Direct use of this solution for the CO2RR → without energy intensive desorber
Spray Coating of Different Materials

SEM of the Electrodes
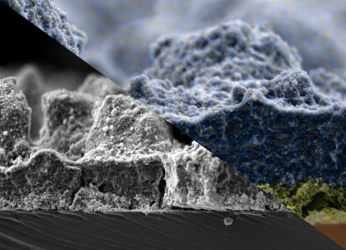
- Custom-made electrodes prepared using spray coating
- Active layer composed of silver nanoparticles
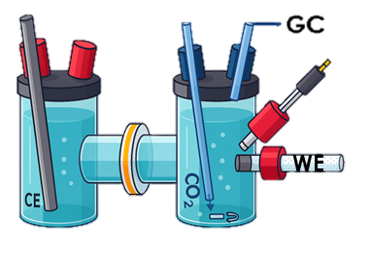
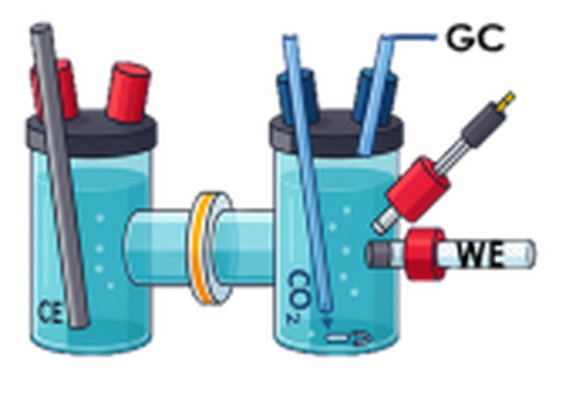
Product Analysis via Gas-Chromatography
- H-cells are employed for fundamental understanding
- Full electrolyzer cells for first step towards practical application
In Collaboration with:

Approach:
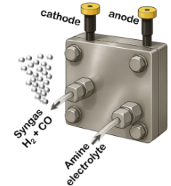
- Catalyst & electrolyte screening
- Deposition & full cell electrolysis
- Upscaling the process
- Screening of different catalysts → high selectivity to formic acid
- Determine the best acidic electrolyte for the CO2 reduction
- Enables rapid screening of catalysts and electrolytes

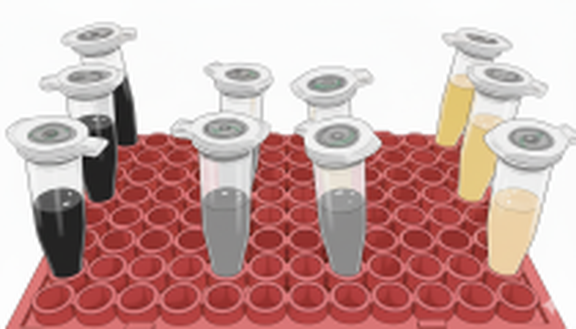
- Optimizing ink composition for deposition
- Implementation of most suitable catalysts into full cell electrolyzer cell (5 cm2)
- Analysis of gaseous and liquid products with gas chromatography
Spraycoater

Electrolyzer Cell

- 5 → 100 → 1000 cm2
- Improve operational parameters
- Investigation of long-term stability

100 cm2

1000 cm2
11.11.2025
New Article Published in ACS Omega
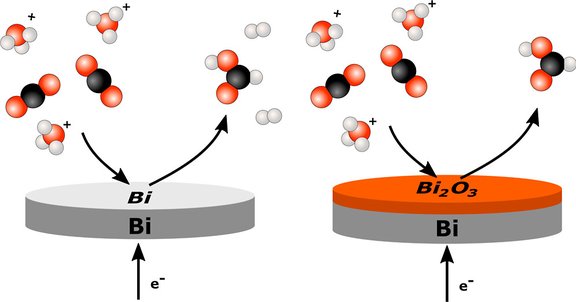
How can CO2 be electrochemically converted to formic acid under industrially relevant, acidic conditions? Our new article "Boosting Formic Acid Production in Mildly Acidic Media - The Role of Native Surface Oxide on the CO2 Reduction Performance" shows that bismuth catalysts achieve over 95 % selectivity toward formic acid at pH 3 while suppressing the competing hydrogen evolution. A naturally formed bismuth oxide layer is found to be key for this performance, paving the way for an implementation of this reaction in proton-exchange membrane systems for industrial application.
Link to the article: https://pubs.acs.org/doi/10.1021/acsomega.5c10136
Photo-electro Integrated Next-Generation energy technologies


Project Phoenix Information
Project Phoenix Information
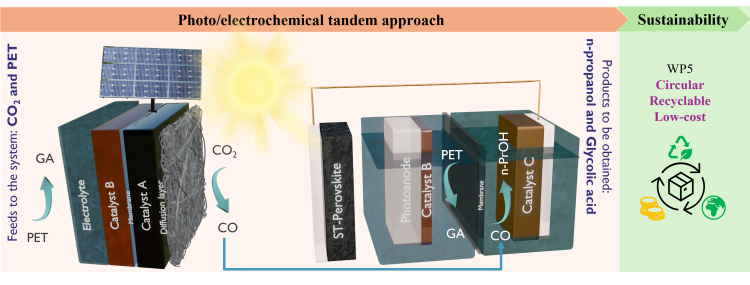
Coupling eCO2 reduction with PET Oxidation in a photovoltaic-electrochemical (PV-EC) device
Approach:
- Solar-driven tandem CO2 conversion paired with PET oxidation for the next generation of renewable energy conversion system
- In-house electrode fabrication using spray-coating
- In-situ characterization for electrocatalyst development
- Catalyst formulation → high selectivity toward CO (cathode) and Glycolic acid (anode-HZB)
- Electrode, membrane and electrolyte screening to optimize outputs
- Long-term stability evaluation under continuous operation




Assessment of assembly architecture and material composition on conversion efficiency, combined with product analysis using gas chromatography (GC), Nuclear Magnetic Resonance (NMR), and High-Performance Liquid Chromatography (HPLC)
Integration of the zero-gap electrolyzer with a CIGS/Perovskite tandem PV module (ZSW) to develop a PV–EC device capable of generating CO and glycolic acid


- Collaborate in developing cost models to optimize the PV-EC system
- Collaborate in assessing the recyclability potential of precious materials within the PV-EC system
In Collaboration with: