Chemical Physics - Group Members
Group leader: Univ.-Prof. Dr. Martin K. Beyer
E-mail: Martin.Beyer@uibk.ac.at
Phone: +43-512-507-52680
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/20
6020 Innsbruck, Austria
Adm. Assistant: Gertrud Sommeregger (temporary)
E-mail: gertrud.sommeregger@uibk.ac.at
Phone: +43-512-507-52681
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/20
6020 Innsbruck, Austria
Senior Scientist: Priv.Doz. Dr. Christian van der Linde
E-mail: Christian.Van-Der-Linde@uibk.ac.at
Phone: +43-512-507-52692
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/21
6020 Innsbruck, Austria
Postdoc: Dr. Marc Reimann
E-mail: Marc.Reimann@uibk.ac.at
Phone: +43-512-507-52694
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/22
6020 Innsbruck, Austria
This FWF-ESPRIT-Project combines theory and experiment to find a generally applicable method for the prediction of spin state energetics in transition metal complexes. These are molecules with a central transition metal ion bonded to surrounding ligands which can be atoms, ions or molecules. A deeper understanding of the spin state energetics is important for the application of metal complexes in catalysis or for efficient storage of information.
The starting point are metal complexes with simple, atomic ligands which are produced by our laser vaporization source and then investigated by UV/VIS spectroscopy. Those data are then simulated by different computational methods. Later on, we will extend our studies to systems with more complex ligands. Some of these molecules will be synthesized in the group of Dr. Moritz Malischewski and transferred into our spectrometer by electrospray ionization.
Postdoc: Dr. Magdalena Salzburger
E-mail: Magdalena.Salzburger@uibk.ac.at
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/17
6020 Innsbruck, Austria
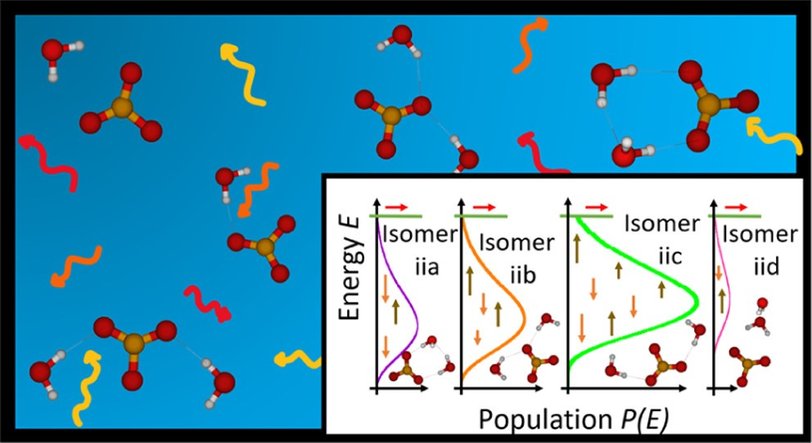
I am currently working on a tutorial review to make the software AWATAR (All wells and all transition states are relevant), developed in our group, accessible to other researchers and students.
AWATAR allows the statistical modelling of processes which change the energy of molecules or molecular clusters by absorption or emission of photons. The uptake of photons may finally lead to dissociation. Examples for dissociation caused by photon absorption, that are experimentally investigated in our group are BIRD (black-body infrared radiative dissociation) and IRMPD (infrared multiple photon dissociation). With AWATAR we gain a deeper understanding of these dissociation processes.
In contrast to other modelling approaches, AWATAR includes several low-lying isomers of the molecule or cluster and every known transition state. This significantly improves the results. A detailed description of the method can be found here.
I am grateful to the FWF for supporting my work within the project "Stability, Structure and Photochemistry of Water Clusters”.
Postdoc: Dr. Shan Jin
E-mail: Shan.Jin@uibk.ac.at
Phone: +43-512-507-52695
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/17
6020 Innsbruck, Austria
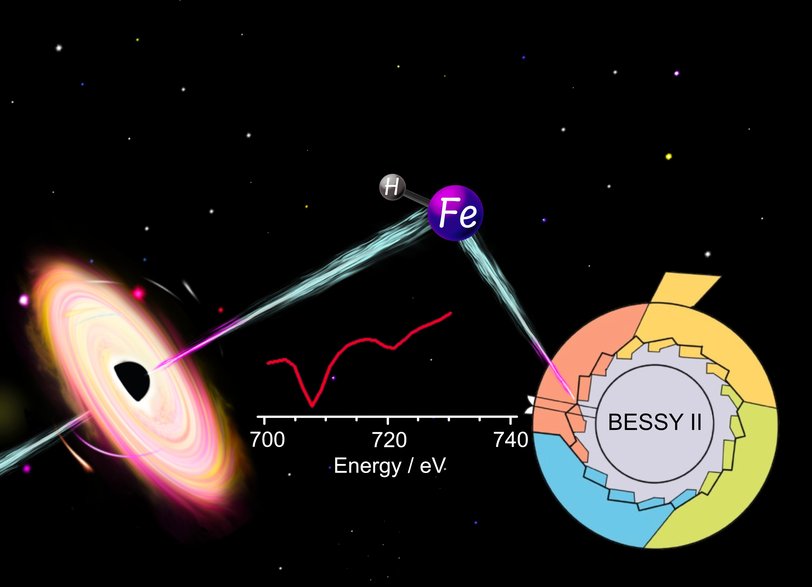
I recently defended my PhD thesis on the spectroscopy of FeH+ and Fe(H2)1,2+. Such complexes are expected to be present in the interstellar medium, but are yet to be identified because of the lack of laboratory data.
To guide an experimental search for these species, I performed infrared multiple photon dissociation spectroscopy (IRMPD) as well as UV/VIS photodissociation spectroscopy.
Together with the group of Tobias Lau @BESSY II, we were also able to gain the first laboratory data of FeH+ that can be directly compared with astronomical observations.
I am grateful for the funding of the FWF for my PhD and the opportunities provided by DK-ALM. Currently, I am a postdoc in the FWF project "Reactivity and Photochemistry of Doped Salt Clusters".
PhD student: Manuel Rainer, M.Sc.
E-mail: Manuel.Rainer@uibk.ac.at
Phone: +43-512-507-52693
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/19
6020 Innsbruck, Austria
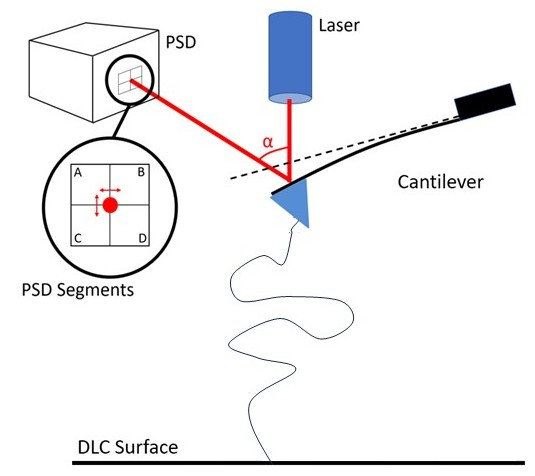
I use an atomic force microscope (AFM) to study reactions induced by applying an external mechanical force to a single molecule chain. The main component of the AFM is the cantilever — a thin, sensitive needle with a sharp pyramidal tip at its end. We chemically functionalize the cantilever, so we can pick up molecules of interest from a special surface on which they were deposited. When a force acts on the tip – for example, during the stretching or rupture of an anchored molecule - the cantilever is bent. This deflection is detected via a laser beam. Using this setup, the rupture force and the length of the molecule chain are obtained.
My most recent project was the investigation of the mechanochemical strength of organometallic rhodium complexes that are used in polymer networks. Their potential applications range from extra stress resistance and self-healing to ultrasound-based drug delivery. I am grateful for the collaboration with the group of Charles E. Diesendruck at Technion who prompted us to investigate the rhodium complexes and synthesizes our polymer compounds.
PhD student: Jessica Hartmann, M.Sc.
E-mail: Jessica.Hartmann@uibk.ac.at
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/22
6020 Innsbruck, Austria
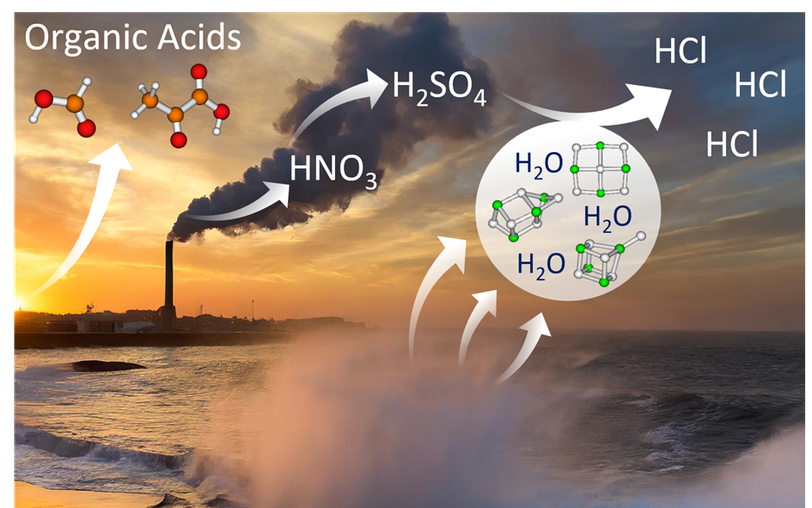
In my thesis I study reactions of cationic and anionic NaCl clusters which serve as model systems for sea-salt aerosols. Sea-salt aerosols are produced from the ocean’s surface and interact with atmospheric trace gases. A better understanding of these reactions is important for modeling global warming processes.
The NaCl clusters are produced via electrospray ionization and the reactions are investigated by means of Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometry. Currently I study acid displacement reactions which lead to the release of hydrogen chloride (HCl). The released HCl contributes to atmospheric acidity and can react further to form other reactive chlorine species which e.g. take part in the ozone depletion in the stratosphere.
To gain as much insight in these processes as possible, quantum chemical calculations are performed, too. On this, I closely collaborate with the group of Chi-Kit Andy Siu in Hong Kong. Thanks to the OeAD I was even able to do a research stay in Hong Kong. I am also grateful to the FWF for supporting my PhD via DK-ALM and the project "Reactivity and Photochemistry of Doped Salt Clusters".
PhD student: Sarah Madlener, M.Sc.
E-mail: Sarah.Madlener@uibk.ac.at
Phone: +43-512-507-52697
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/17
6020 Innsbruck, Austria
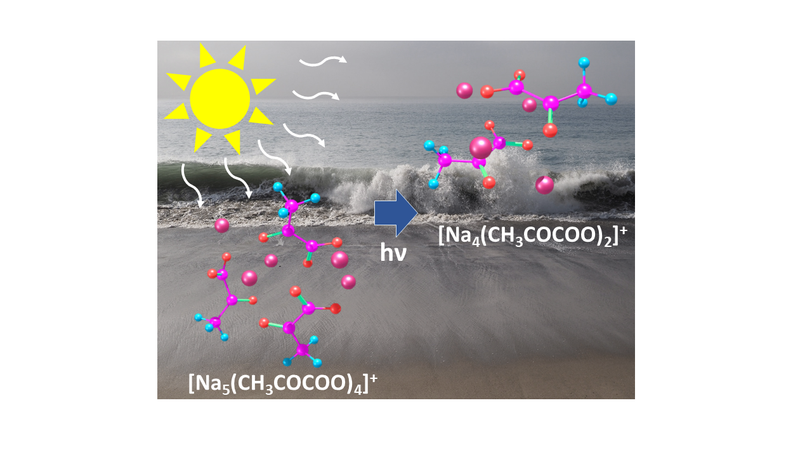
I study UV/VIS spectra of doped sea-salt clusters which serve as model systems for marine aerosols. Aerosols are tiny particles in the air that play an important role in the earth’s climate. Since the oceans cover more than 70% of the surface of our planet, many of these aerosols are created when waves break and sea spray is released into the air. Marine aerosols contain not only sea salt but also many different organic substances. Strong sunlight can change this organic material through complex chemical reactions. Marine aerosols can also take up trace gases such as nitrogen oxides, which then may as well react under the influence of sunlight.
To reduce the complexity of these reactions I produce anionic or cationic salt clusters doped with organic acids or nitrogen oxides by means of an electrospray ionization source (ESI). These clusters are then transferred in the ultra-high vacuum region of our 9.4 T FT-ICR mass spectrometer. There, I can mass select individual cluster sizes and store these clusters for up to 20s. During this time, the clusters are irradiated at different wavelengths by a tunable laser system. This way, I can distinguish between wavelength-dependent reaction pathways of different cluster sizes.
I am grateful to the FWF for supporting my PhD via DK-ALM and the project "Reactivity and Photochemistry of Doped Salt Clusters".
PhD student: Dominik Jank, M.Sc.
E-mail: Dominik.Jank@uibk.ac.at
Phone: +43-512-507-52698
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/22
6020 Innsbruck, Austria
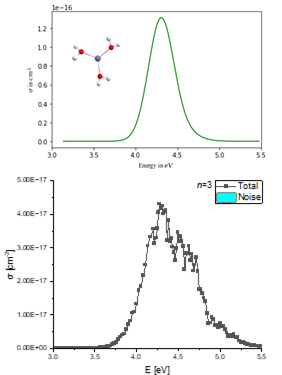
Hydrogen plays a key role in making the energy, transport, and chemical sectors carbon-neutral. To improve the efficiency of hydrogen generation it is important to understand the mechanisms of the hydrogen evolution reaction in electrochemical cells. These molecular processes can be quite complex. Therefore, gas phase models like hydrated metal ions are investigated.
In my thesis I study the UV/VIS spectra and photochemistry of Zn+(H2O)n, Zn2+(H2O)n and Al+(H2O)n, with up to 15 water molecules. These clusters are known to eliminate atomic or molecular hydrogen upon irradiation with ultraviolet light. I investigate this photochemical hydrogen evolution reaction in the laboratory with size-selected clusters. With a tunable laser system, I can probe the efficiency of hydrogen evolution at different wavelengths. The clusters are small, so that a numerical description of the quantum states of the system is possible. This way, I can simulate the absorption spectrum and understand how the hydrogen atoms or molecules are formed in this photochemical reaction.
I am contributing to the FWF project "Reactivity and Photochemistry of Doped Salt Clusters".
Master student: Sarah Keiler, B.Sc.
E-mail: Sarah.Keiler@student.uibk.ac.at
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/17
6020 Innsbruck, Austria
My Master's Thesis deals with photodissociation spectroscopy of ionic metal compounds which could be important in astrochemistry. Many new molecules in the interstellar medium wait for their identification, so that physical and chemical processes, leading e.g. to formation of stars or planets, can be modeled with higher confidence. My work focusses on the Diffuse Interstellar Bands (DIBs), which are broad absorption bands in the near-infrared and visible region of the electromagnetic spectrum. In my thesis, I want to analyze molecules that could be responsible for these kinds of absorptions, so called “DIB carriers”.
The starting point of my research are small cationic magnesium oxide clusters MgnOm+ which I want to produce by means of our electrospray ionization source (ESI) and detect via mass spectrometry with our ion trap. For comparison with astronomical observations, I want to record their spectroscopic fingerprints. This approach could help reveal whether some of these ions exist in the interstellar medium and might be the carriers of some DIBs.
Master student: Claus Schwendinger, B.Sc.
E-mail: Claus.Schwendinger@student.uibk.ac.at
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/17
6020 Innsbruck, Austria
Master student: Simon Bihler, B.Sc.
E-mail: Simon.Bihler@student.uibk.ac.at
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/17
6020 Innsbruck, Austria
The interstellar medium (ISM) contains substantial amounts of iron, produced mainly by massive stars and supernova explosions. However, only a small fraction of the iron is found as neutral atoms or atomic ions in astronomical observations. Possible sinks are oxide and hydroxide species.
That is why I investigate the photodissociation spectroscopy of small cationic iron oxide/hydroxide clusters in my Master's thesis. The starting point of my research are FexOy+ ions, which I plan to produce by means of our electrospray ionization source (ESI) or laser vaporization. These clusters will be transferred in the ultra-high vacuum region of our 9.4 T FT-ICR mass spectrometer, e.g., stored and irradiated at different wavelengths by a tunable laser system. The recorded photodissociation spectra can then be compared to spectra observed by astronomers.
Master student: Sebastian Fabian Wörz, B.Sc.
E-mail: Sebastian.Woerz@student.uibk.ac.at
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/19
6020 Innsbruck, Austria
Nitrophenols are found in air, water and soil. Their main source is exhaust from cars and other combustion processes like biomass burning. It is known that sunlight causes photochemical reactions in nitrophenols. A potential product is nitrous acid (HONO), which plays a significant role in atmospheric chemistry. The details of the reaction mechanism are still unclear. This is why I want to study the photodissociation spectra of nitrophenols and nitrophenolates, the deprotonated form, in my Master’s thesis. I have started with the investigation of 3-nitrophenolate and plan to examine the isomers 2-nitrophenolate and 4-nitrophenolate as well. Later on, I want to extend my research on the photochemistry of sea salt clusters doped with a nitrophenol molecule. It is known that nitrophenols are photoacids. Therefore, irradiation with light may cause the release of HCl from sea-salt clusters that are doped with neutral nitrophenol molecules. Given the high abundance of nitrophenols in the atmosphere, such a reaction could be a significant source of HCl. The doped sea salt clusters will be produced by means of an electrospray ionization source (ESI), stored in an ion trap or FT-ICR mass spectrometer, and irradiated at different wavelengths by a tunable laser system. The products are in both cases detected via mass spectrometry. With quantum chemical calculations, I want to get a deeper understanding of the observed processes.
Master student: Ekaterina Tatarnikova, B.Sc.
E-mail: Ekaterina.Tatarnikova@student.uibk.ac.at
Address:
Universität Innsbruck, Institut für Ionen- und Angewandte Physik
Technikerstrasse 25, Room 3/22
6020 Innsbruck, Austria
Sea-salt aerosols form at the ocean surface and interact with trace gases in the atmosphere. Improved knowledge of these processes is essential for reliable global-warming modeling. Since the composition of sea-salt aerosols can be quite complex due to the uptake of trace gases such as nitrogen oxides and sulfur dioxide, doped salt clusters are used as model systems.
In my Master’s thesis, I investigate the reactivity of cationic sodium nitrate (Nax(NO3)y+) and sodium sulfate clusters (Nax(SO4)y+) as well as their chlorine-doped mixtures (Nax(NO3)yClz+ and Nax(SO4)yClz). While large amounts of sulfate ions are present in sea salt, nitrate accumulates in sea-salt aerosols in the atmosphere by pickup of nitric acid and release of hydrogen chloride. I will examine how the reactivity of these clusters depends on cluster size, composition and structure. The measurements are performed with FT-ICR mass spectrometry which allows reaction times of up to one minute and size selection of clusters, if necessary. Atmospherically relevant reactants such as formic acid or trifluoracetic acid will be used. Formic acid is formed in the atmosphere by photochemical oxidation of volatile organic compounds, trifluoracetic acid from the breakdown of certain fluorinated compounds, like hydrofluorocarbons used in refrigerants.














