Research
Research in the Dielmann group covers diverse topics in the field of molecular inorganic chemistry and homogeneous catalysis. We design and synthesize reactive main group species and transition metal complexes for energy-efficient catalytic conversions of particularly inert molecules (e.g. CO2, SF6, H2, N2, SO2) and strong chemical bonds. Our research strategy aims at an advanced understanding of bond activation mechanisms achieved by combining experimental, spectroscopic and computational methods. Fueled by new discoveries from basic research, we aim to contribute to important scientific challenges through innovative molecular design and the development of new synthetic methods. A unifying theme of our molecular design is the strategic utilization of strong substituent effects to predictable control molecular properties and to elevate them to extreme levels. In this context, present research activities are concerned with the synthesis, coordination chemistry and application of new ligands equipped with N-heterocyclic imine (NHI) substituents. In particular, electron-rich phosphines and pyridines are the focus of interest. The strong donating ligands can be used as ligands in homogeneous catalysis and in new molecular materials with interesting photophysical properties.
Current research projects are concerned with
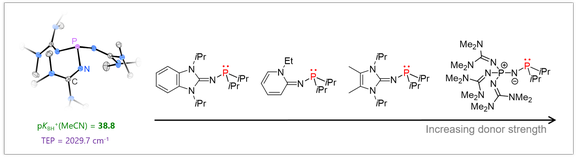
Superbasic phosphines:
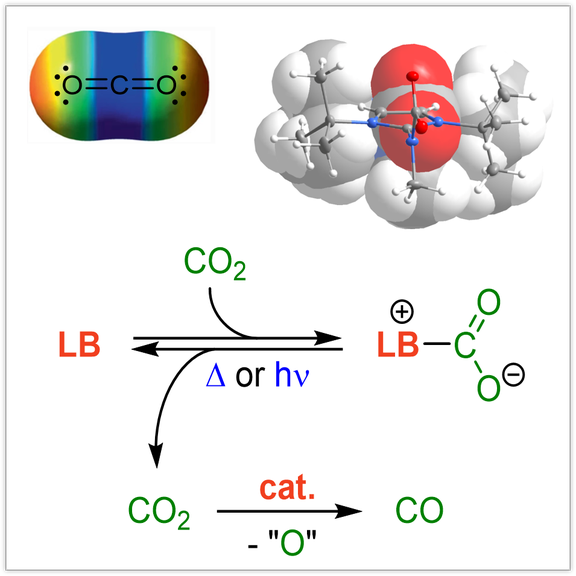
Tertiary phosphines are widely used in many areas of synthetic chemistry with profound impact on organic synthesis, coordination chemistry and catalysis. We aim at expanding the accessible stereoelectronic properties of phosphines beyond their classical boundaries to provide new opportunities in phosphine-mediated transformations and catalysis. As a starting point, we discovered that the electron-donating ability of phosphines can be significantly enhanced by equipping them with strong π-donor substituents such as imidazoline-2-ylidenamino groups. The ability of these substituents to act as π-donor towards phosphorus has a significant influence on the phosphine’s electronic properties. As a consequence, phosphines equipped with three donor groups are ranked amongst the strongest nonionic Brønsted and Lewis bases. The novel phosphines are promising ligands in organometallic catalysis. Moreover, they were used for the reversible complexation and cleavage of CO2, the generation of sulfur monoxide from sulfur dioxide, and the selective fragmentation of SF6.
Selected Publications:
Angew. Chem. Int. Ed. 2015 | Chem. Eur. J. 2017 | Organometallics 2019 | Chem. Eur. J. 2020 | Angew. Chem. Int. Ed. 2022
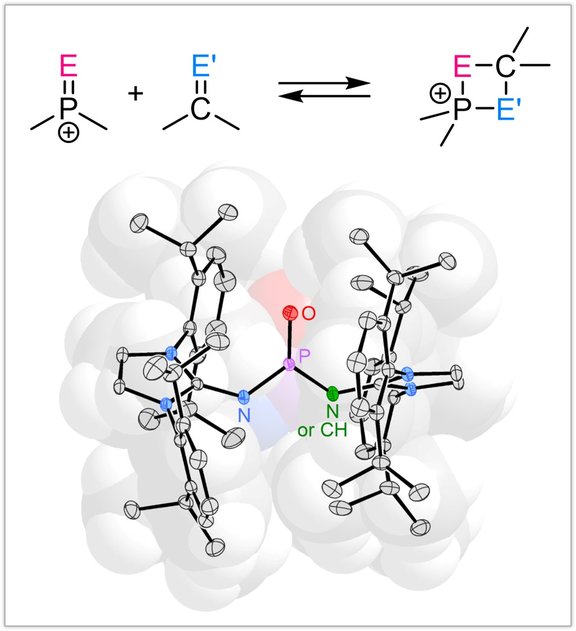
Low-coordinate phosphorus species:
We are interested in the synthesis of novel main group species and explore new concepts to predictable control their molecular properties. By exploiting strong π donor substituents, we stabilized molecular Lewis acids, such as chalcogenophosphonium and phosphorandiylium ions, with the goal to apply them in main group element catalysis and bond heterolysis reactions.
Selected Publications:
Angew. Chem. Int. Ed. 2018 | J. Am. Chem. Soc. 2020 | Nat. Chem. 2019
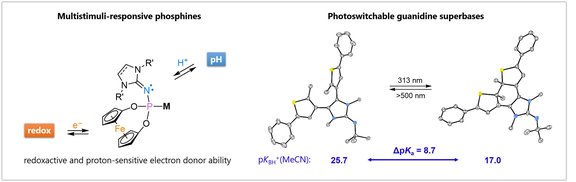
Strongly donating, switchable ligands:
Design, modification and tailoring of ancillary ligands are key tools to tune the activity, productivity and selectivity of homogeneous catalysis. We use stereoelectronically variable N-heterocyclic imine (NHI) substituents to tune the properties of privileged ligand scaffolds.. The basic NHIs are also utilized in the context of metal-ligand cooperation and to introduce stimuli-responsive components into our ligands.
Selected Publications:
Chem. Eur. J. 2021 | Angew. Chem. Int. Ed. 2022
CO2 capture and conversion:
Selective and reversible capture of CO2 either from flue gas or directly from air and its transformation into value-added chemicals, materials and fuels is one of the most important goals to mitigate CO2 accumulation in our atmosphere. In this context, we explore different strategies to activate CO2 in an energy-efficient manner including the formation of reversible Lewis base adducts, composite systems for electrochemical CO2 reduction and photo-triggered CO2 capture-and-release systems. The conversion of CO2 to CO using molecular systems is also under investigation in our laboratories.
Selected Publications:
J. Am. Chem. Soc. 2016 | Green Chem. 2019 | ACS Sustainable Chem. Eng. 2021
SF6 activation and derivatization:
Sulfur hexafluoride is a fascinating molecule with physicochemical properties that are distinct from that of all other sulfur halides, as well as other gases. It is therefore used in a number of industrial applications and processes, although it is recognized as the most potent greenhouse gas in the atmosphere. Due to the extreme chemical inertness of the octahedral molecule, methods involving the direct fragmentation of SF6 require forcing conditions leading to high energy consumption and the formation of toxic and corrosive products. The chemical valorization of SF6 under mild conditions has therefore garnered considerable attention. Our research interests interest is to develop user-friendly methods for the chemical activation of SF6, which will ultimately open up new avenues for SF6 derivatization.
Selected Publications:
Angew. Chem. Int. Ed. 2018| Angew. Chem. Int. Ed. 2022 | Green Chem. 2022

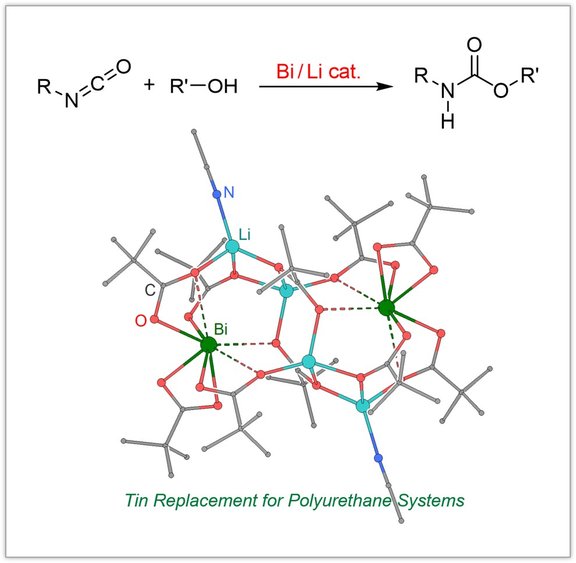
Polyurethane catalysts:
Polyurethanes (PUs) represent one of the most important classes of polymer materials with numerous technical applications, including elastomers, rigid foams, soft foams, coatings and adhesives. Organotin compounds are important catalysts in aliphatic polyurethane synthesis due to their high catalytic activity and advantageous properties. Due to their toxicity, however, it has been a long-standing goal to develop more environmentally benign catalysts. In this context, we develop catalysts for PU applications based on main group elements. For instance we showed that the bismuth-catalyzed urethane reaction is significantly accelerated by generating heterobimetallic mixtures of bismuth and lithium carboxylates.
Selected Publications: