Cornexistin is produced in nature by the fungus Paecilomyces variotii and acts as a strong broad-spectrum herbicide against agricultural weeds. In cooperation with Bayer Crop Science, Thomas Magauer and his team succeeded in a four-year project to realize the world's first synthesis of this natural substance. The use of a natural compound in agriculture has many advantages over fully synthetic compounds whose effect on other organisms, such as bees, is still unclear. With their research, chemists are helping to circumvent this problem. "Even though the exact mechanism of action of cornexistin is still unclear, it is already known that corn is resistant to it. The typical weeds are reduced in the field, while the maize survives and the yield can be increased," says Magauer. Once the mechanism of action has been clarified, the scientists might also work on designing other artificial compounds that have a similar or even better effect. It would also be conceivable to produce a simplified modification of cornexistin. "There is some evidence that cornexistin inhibits asparatate amino transferase, an enzyme that is essential for plants," explained the chemist.
Complex architecture
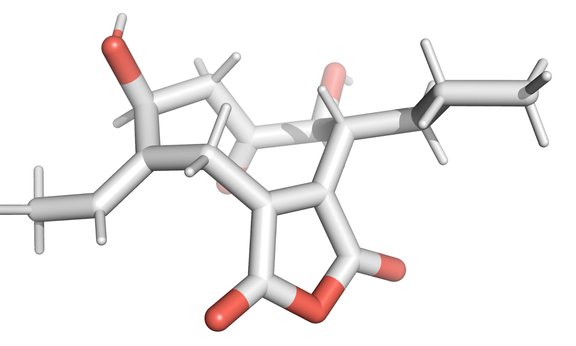
The molecular structure of cornexistin poses a major synthetic challenge for chemists. "The compounds are very complex. A carbocyclic nine-ring, which is difficult to access, is attached to a very unstable maleic acid unit. In addition, the basic framework has several positions functionalized with oxygen that can be degraded very easily," says the head of the research group. Besides the challenge of constructing the nine-membered ring, there are other sources of instability. The compound features a delicate maleic anhydride motif. "These are carboxylic acids from which water has been removed and are therefore very reactive. As soon as the compound is exposed to traces of water, it can undergo rapid hydrolysis," said Magauer explaining some of the difficulties. Several attempts have been made worldwide to synthesize cornexistin, but all strategies have failed in the past. "The fermentative production and clarification of the biosynthesis was realized by the Cox group in Hanover. The developed synthesis is a valuable alternative that also allows the introduction of specific structural modifications that would not be possible via semi-synthesis or fementation," explains Thomas Magauer. The team was not only able to produce the desired natural substance in enantiomerically pure form, but also the mirror image form as well as numerous unnatural analogues. " These compounds will now be further investigated to elucidate the exact mechanism of action as well as study the biological activity of the compound library," the chemist states. The results of the research were published in the journal "Angewandte Chemie".
Publication: Total Synthesis of (+)‐Cornexistin. Christian Steinborn, Raphael Wildermuth, David Barber, Thomas Magauer.

